malaria vaccine trials
Earlier research found that the vaccine was safe and protected healthy US. The approach is now being tested in a Phase 2 clinical trial in Mali.

Efficacy Of A Low Dose Candidate Malaria Vaccine R21 In Adjuvant Matrix M With Seasonal Administration To Children In Burkina Faso A Randomised Controlled Trial The Lancet
Malaria Vaccination with or without Chemoprevention This trial assessed the efficacy of the vaccine RTSSAS01E as compared with chemoprevention in preventing malaria.

. CDC Statement on Results from RTSSAS01 Malaria Vaccine Trial. What is a Phase 3 clinical trial. A vaccine against malaria has shown promise in early clinical trials raising hopes that it might one day prove to be an effective weapon against one of.
Antimalarial drugs and measures to control mosquitoes have reduced the malaria burden worldwide. Clinical trials are a critical source of data for decisions around the development of vaccines drugs and other medical interventions. June 30 2021 Two US.
Authors Brian Greenwood 1 Pedro Alonso. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were later exposed to disease-causing malaria parasites. Research continues with other malaria vaccines.
The trial was mainly funded by a European and Developing Countries Clinical Trials Partnership grant to the Multi-Stage Malaria Vaccine Consortium grant agreement RIA2016V-1649 with additional support from the Wellcome Trust through Translation Award 205981Z17Z and from the UK National Institute for Health Research to the Oxford. But this decline has stalled in recent years. This is a randomized double blind placebo-controlled single site trial of a condensed regimen of PfSPZ Vaccine administered on Days 1 8 and 29 by direct venous inoculation DVI to assess safety tolerability immunogenicity and vaccine efficacy VE against heterologous controlled human malaria infection CHMI conducted at varying time intervals.
To determine whether processing of the circumsporozoite protein as a component of the RTSS particulate antigen yields the same HLA-DR-restricted ep. The first malaria vaccine RTSS now enters pilot implementation in three African countries. Affiliation 1 Department of Infectious and.
Vivax remains a key priority. These pilot implementation studies are being initiated in Kenya Malawi and Ghana to inform the broader roll-out recommendation. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were.
Phase 3 trial results for vaccine RTSSAS01 7 April 2017 QA. The Centers for Disease Control and Prevention welcomes the announcement today that results from the clinical trial in Africa of a malaria vaccine candidate show it prevented about half of malaria cases including the most severe in young children. This is an exciting time for malaria vaccine development with a number of promising clinical trials recently completed and others currently underway although there.
Tuesday October 18 2011. A malaria vaccine is a vaccine that is used to prevent malariaThe only approved vaccine as of 2021 is RTSS known by the brand name Mosquirix. A malaria vaccine as part of the integrated malaria control and elimination efforts will have a major impact on public health in sub-Sahara Africa.
Researchers developed a malaria vaccination strategy that provided broad long-lasting protection in controlled clinical trials. Although there were fewer cases in. The RTSS vaccine reduced clinical and severe cases of malaria by about one-third in 517-month-old children over four years who received the three.
Malaria vaccine trials Chem Immunol. Final results of a phase 3 individually randomised controlled trial. Efficacy and safety of RTSSAS01 malaria vaccine with or without a booster dose in infants and children in Africa.
The vaccine combines live parasites with either of two widely used antimalarial drugsan approach termed chemoprophylaxis. An efficacious malaria vaccine would be an important tool to combat the enormous socioeconomic burden caused by malaria. In December 2016 an expert meeting was convened at NIAID NIH in Rockville Maryland to deliberate on the rationale and design of malaria vaccine trials in pregnant women.
They are carried out in several phases. Were a promising advance in development of a malaria vaccine for African children. The IOM formed a committee of 11 experts with collective expertise in malaria vaccine research parasite immunology malarial biology clinical trials and regulatory affairs industrial and public-sector vaccine development biologic products research and development vaccinology military research and development programs tropical medicine.
Consultation on malaria vaccines and biologicals research and development 15-16 July 2019 Salle C WHO Geneva Switzerland In 2017 malaria caused an estimated 219 million cases and 435 000 deathsillness. It requires four injections. Extended Follow-Up Following a Phase 2b Randomized Trial of the Candidate Malaria Vaccines FP9 ME-TRAP and MVA ME-TRAP among Children in Kenya Philip Bejon 1 Edna Ogada 1 Tabitha Mwangi 1 2 Paul Milligan 3 Trudie Lang 2 Greg Fegan.
Decades of endeavor have taught that achieving this goal will be challenging. RTSSAS01 is the first malaria vaccine to be tested in Phase 3 clinical trials and the first to be assessed in routine immunization programs in malaria-endemic areas. Phase 3 trial results for vaccine RTSSAS01.
In a Phase 2a trial of the RTSSAS vaccine we described significant association between protection against infection and vaccine-induced CD4 T cells. Investigators further assessed safety and studied whether the vaccine could protect adults against naturally occurring malaria infection in a. The protective efficacy of RT.
However recent innovation in malaria vaccine research and a diverse pipeline of novel vaccine candidates for clinical. CDC in collaboration with the Kenya Medical Research Institute led the trial at one site in western Kenya. While malaria vaccine development has made significant progress in recent years no trials of malaria vaccines have ever been conducted in pregnant women.
Adults who hadnt been previously exposed to malaria. The development of highly effective and durable vaccines against the human malaria parasites Plasmodium falciparum and P. Following decades of research the officials at the World Health Organization recently recommended the widespread use of the RTSSAS01 malaria vaccine for.
A vaccine against malaria has been shown to be highly effective in trials in Africa holding out the real possibility of slashing the death toll. The most effective malaria vaccine is R21Matrix-M with a 77 efficacy rate shown in initial trials and significantly higher antibody levels than with the.

World S First Malaria Vaccine Given Go Ahead For Immunisation Of African Children Bbc Science Focus Magazine

Vaccines For Malaria In Clinical Trial Phase Download Scientific Diagram

R21 Matrix M Malaria Vaccine Becomes First To Reach 75 Efficacy Target
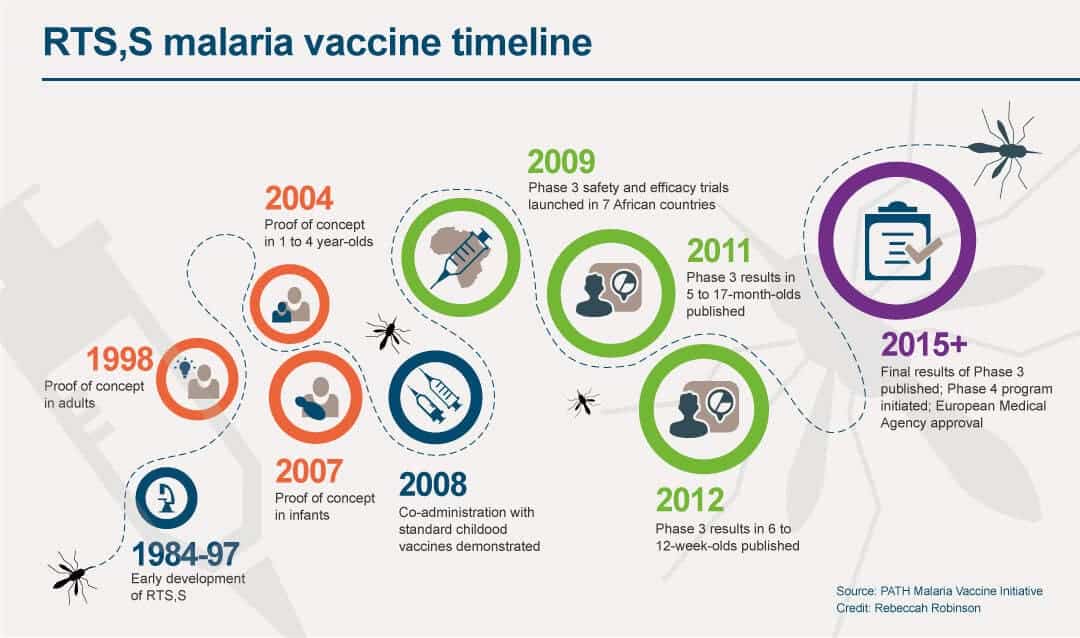
World S First Malaria Vaccine Launched In A Pilot Program
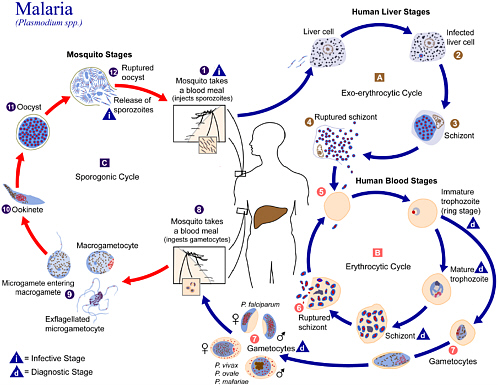
3 Malaria Vaccines Battling Malaria Strengthening The U S Military Malaria Vaccine Program The National Academies Press
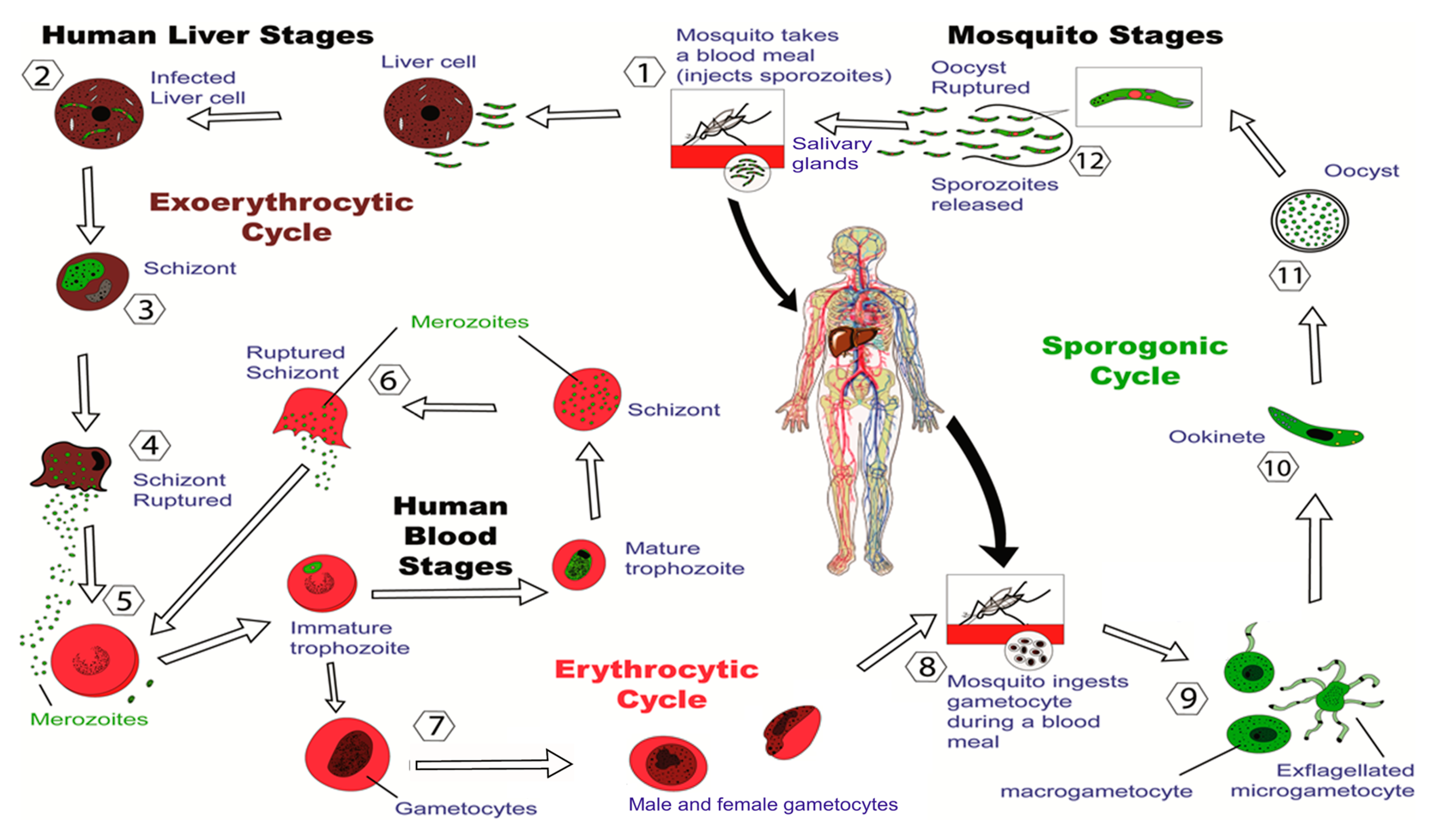
Vaccines Free Full Text Progress In The Development Of Subunit Vaccines Against Malaria Html
Malaria Vaccine Found Safe And Protective National Institutes Of Health Nih

Malaria Vaccine Candidates In Clinical Development Data Sources For Download Scientific Diagram
Schedule On The Malaria Vaccine Development For Approximately 50 Years Download Scientific Diagram

Malaria Vaccines Malaria Site

Cryoport Developing Cold Chain Logistic Models For Malaria Vaccine

Oxford Malaria Vaccine Proves Highly Effective In Burkina Faso Trial Malaria The Guardian

Current Malaria Vaccines Under Preclinical Development Or In Clinical Download Table

World S First Malaria Vaccine Gets Who Recommendation Goats And Soda Npr
A Malaria Vaccine With Live Parasites Shows Promise In A Small Trial Science News